Chemical Structure and Properties of Lithium Dodecyl Sulfate (LDS) vs Sodium Dodecyl Sulfate (SDS)
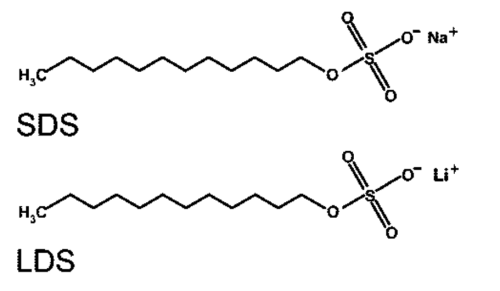
When comparing Lithium Dodecyl Sulfate (LDS) to Sodium Dodecyl Sulfate (SDS), it’s essential to delve into their chemical structures and properties to understand their similarities and differences.
- Chemical Structure:
Sodium Dodecyl Sulfate (SDS):
Chemical Formula: C₁₂H₂₅NaO₄S
SDS, also known as sodium lauryl sulfate (SLS), consists of a dodecyl (C₁₂H₂₅) hydrophobic alkyl chain attached to a hydrophilic sulfate group (SO₄²⁻). The sodium ion (Na⁺) is the counterion.
Lithium Dodecyl Sulfate (LDS):
Chemical Formula: C₁₂H₂₅LiO₄S
LDS has a similar structure to SDS, with the key difference being the counterion. Instead of sodium, lithium (Li⁺) serves as the counterion in LDS.
- Physical Properties:
Solubility:
SDS: Highly soluble in water, forming clear solutions. Widely used because of its ability to dissolve in both warm and cold water.
LDS: Also soluble in water, but the solubility may vary slightly due to the different ionic radius and hydration properties of lithium compared to sodium.
Melting Point:
SDS: The melting point of SDS is around 206-208°C.
LDS: The melting point may differ due to the presence of lithium, but specific data might be less commonly reported and could require experimental determination.
Critical Micelle Concentration (CMC):
SDS: SDS has a well-documented CMC of about 8.2 mM in water at 25°C. This value is essential for understanding its behavior as a surfactant.
LDS: The CMC for LDS might be different due to the lithium ion’s effect on micelle formation. Surfactants with different counterions can exhibit variations in CMC values.
- Ionic Characteristics:
Ionic Size and Charge Density:
Sodium (Na⁺): Sodium ions have a larger ionic radius than lithium and a lower charge density.
Lithium (Li⁺): Lithium ions are smaller and have a higher charge density, which can influence the surfactant’s behavior in solution, potentially affecting micelle formation and the interaction with other molecules.
- Effect on Micelle Formation:
SDS: The micelles formed by SDS are well-studied, typically spherical in shape, and are effective in solubilizing hydrophobic molecules in aqueous environments.
LDS: The smaller lithium ion may lead to differences in micelle structure and stability. This could potentially result in variations in the size, shape, and aggregation number of the micelles.
- Interaction with Biomolecules:
SDS: Widely used in protein denaturation, especially in SDS-PAGE, due to its ability to uniformly coat proteins and give them a negative charge.
LDS: Its interaction with proteins and other biomolecules might differ slightly due to the lithium ion, potentially impacting its effectiveness in certain applications.
In summary, while LDS and SDS share a similar dodecyl sulfate backbone, the substitution of sodium with lithium introduces variations in their physical properties and behavior in solution. Understanding these differences is crucial for selecting the appropriate surfactant for specific applications and can provide insights into the design of new surfactant systems.