Both sodium C14-16 olefin sulfonate and sodium lauryl sulfate are surfactants commonly used in personal care and cleaning products, but they have some differences:
- Chemical structure:
- Sodium C14-16 olefin sulfonate: Derived from long-chain olefins
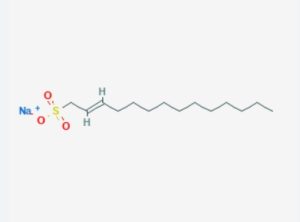
This is an α-olefin sulfonate derived from long-chain α-olefins (typically 14 to 16 carbon atoms) through a sulfonation reaction.
Its molecular structure includes a long hydrocarbon chain (14-16 carbons), a double bond, and a sulfonate group (-SO3-).
The structural formula can be simplified as R-CH=CH-(CH2)n-CH2-SO3Na, where R is an alkyl chain, and n depends on the total number of carbon atoms.
Due to the diversity of olefin raw materials, the actual product is usually a mixture of molecules with different chain lengths.
- Sodium lauryl sulfate: Derived from lauryl alcohol
This is an alkyl sulfate made from lauryl alcohol (a 12-carbon straight-chain alcohol) through sulfation and neutralization.
Its molecular structure consists of a 12-carbon straight alkyl chain connected to a sulfate ester group (-OSO3-).
The structural formula can be represented as CH3-(CH2)11-OSO3Na.
Compared to olefin sulfonate, its structure is more uniform and well-defined.
These structural differences lead to distinct characteristics in performance and application. Olefin sulfonate, due to its structural diversity, typically exhibits milder properties, while lauryl sulfate, with its simpler structure, demonstrates stronger cleaning power.
- Mildness:
- Sodium C14-16 olefin sulfonate: Generally considered milder
Generally considered milder on skin and hair
Less likely to cause irritation or dryness
Better suited for sensitive skin formulations
The presence of a double bond in its structure may contribute to its gentler nature
Its mixed molecular composition can lead to a more balanced interaction with the skin
- Sodium lauryl sulfate: Can be more irritating to skin and eyes
Known for being more harsh and potentially irritating
Can strip natural oils from skin and hair more aggressively
May cause dryness, redness, or itching in some individuals
Its strong cleansing power can disrupt the skin’s natural barrier
More likely to cause eye irritation in shampoos and face washes
Factors contributing to the difference in mildness:
Molecular structure: The more complex structure of olefin sulfonate interacts less aggressively with skin proteins.
pH: SLS tends to have a higher pH, which can be more disruptive to the skin’s natural acidity.
Penetration: SLS molecules can penetrate deeper into the skin, potentially causing more irritation.
Defatting action: SLS is more effective at removing oils, which can lead to excessive drying.
Due to these differences, products formulated for sensitive skin or marketed as “gentle” often prefer sodium C14-16 olefin sulfonate over sodium lauryl sulfate. However, the overall mildness of a product also depends on its total formulation, not just the choice of primary surfactant.
- Cleansing power:
- Sodium C14-16 olefin sulfonate: Good cleansing ability
Good cleansing ability
Effective at removing dirt, oil, and other impurities
Produces a moderate amount of foam
Maintains its effectiveness in hard water
Works well in a wide range of pH levels
- Sodium lauryl sulfate: Excellent cleansing and foaming properties
Excellent cleansing and foaming properties
Very effective at removing oils and dirt
Produces abundant, stable foam
May be less effective in hard water
Works best in slightly acidic to neutral pH ranges
Comparison of cleansing properties:
Dirt removal: Both are effective, but SLS is generally more aggressive.
Oil removal: SLS is superior in removing oils, which contributes to its stronger cleansing power.
Foam production: SLS produces more foam, which consumers often associate with better cleaning.
Rinsability: Olefin sulfonate typically rinses off more easily than SLS.
Residue: SLS may leave more residue on surfaces, which can be perceived as a “squeaky clean” feeling.
Factors affecting cleansing power:
Molecular structure: SLS’s simpler structure allows it to penetrate and lift dirt more easily.
Critical micelle concentration: SLS has a lower CMC, allowing it to form micelles more readily, enhancing its cleansing ability.
Interaction with hard water: Olefin sulfonate performs better in hard water conditions.
Applications:
SLS is often chosen for products where strong cleansing is priority (e.g., dish soaps, heavy-duty shampoos).
Olefin sulfonate is preferred in formulations balancing cleansing power with gentleness (e.g., body washes, mild shampoos).
It’s worth noting that while SLS has superior cleansing power, this can sometimes be a drawback in personal care products as it may be too harsh for regular use on skin and hair.
- Environmental impact:
- Sodium C14-16 olefin sulfonate: Often considered more biodegradable
Often considered more biodegradable
Lower aquatic toxicity compared to SLS
Breaks down more readily in wastewater treatment processes
Produces fewer byproducts during degradation
Generally considered to have a lower environmental footprint
- Sodium lauryl sulfate: Biodegradable but may have higher aquatic toxicity
Biodegradable, but may have higher aquatic toxicity
Can persist longer in aquatic environments
May have more significant effects on aquatic organisms
Potential to cause foaming in waterways if released in large quantities
Production process may have a higher environmental impact
Comparison of environmental factors:
Biodegradability: Both are biodegradable, but olefin sulfonate typically degrades faster and more completely.
Aquatic toxicity: SLS has been shown to have higher toxicity to some aquatic organisms.
Bioaccumulation: Neither tends to bioaccumulate significantly in organisms.
Eutrophication potential: SLS may contribute more to eutrophication due to its sulfate content.
Production impact: The manufacturing process for SLS often requires more energy and resources.
Regulatory considerations:
Many countries have stricter regulations on SLS use in products that are likely to enter waterways.
Olefin sulfonate is often preferred in “eco-friendly” or “green” product formulations.
Long-term environmental effects:
While both substances eventually break down, the long-term effects of continuous release into the environment are still being studied.
The impact on marine ecosystems is of particular concern, especially for products like shampoos and soaps that are directly washed down drains.
It’s important to note that the environmental impact of these surfactants can vary depending on concentration, usage patterns, and local wastewater treatment capabilities. Additionally, the overall environmental impact of a product depends on many factors beyond just the choice of surfactant.
- Cost:
- Sodium C14-16 olefin sulfonate: Generally more expensive
Generally more expensive
Higher production costs due to more complex manufacturing process
Price can fluctuate based on availability of raw materials (olefins)
Often considered a premium ingredient in formulations
May increase the overall cost of the final product
- Sodium lauryl sulfate: Usually less expensive
Usually less expensive
Lower production costs due to simpler manufacturing process
More stable pricing due to widespread availability and production
Often used as a cost-effective surfactant in mass-market products
Helps keep the overall product cost down
Factors influencing cost:
Raw materials: Olefins used for olefin sulfonate are generally more expensive than lauryl alcohol used for SLS.
Manufacturing process: The sulfonation of olefins is more complex and costly than the sulfation of lauryl alcohol.
Scale of production: SLS is produced in larger quantities, benefiting from economies of scale.
Market demand: Higher demand for gentler surfactants has increased the price of olefin sulfonate.
Purity and quality: Higher purity grades of both surfactants come at a premium price.
Impact on product formulation:
Formulators often need to balance cost with performance and marketing claims.
Higher-end or specialty products are more likely to use olefin sulfonate despite the cost.
Mass-market products often opt for SLS to maintain competitive pricing.
Cost-effectiveness considerations:
While SLS is cheaper, its harsher nature may require additional ingredients to mitigate its effects, potentially offsetting some of the cost savings.
The higher cost of olefin sulfonate may be justified in products targeting sensitive skin or making “gentle” claims.
Market trends:
Growing consumer awareness of ingredients has led to increased demand for alternatives to SLS, potentially affecting future pricing.
Sustainability concerns may impact the cost of both surfactants in the long term.
It’s worth noting that while cost is an important factor, it’s often balanced against performance, consumer preferences, and marketing positioning when choosing between these surfactants for a particular product formulation.
- Usage:
Both are used in shampoos, body washes, and other personal care products, but sodium C14-16 olefin sulfonate is often preferred in more gentle formulations.
Sodium C14-16 Olefin Sulfonate:
Often preferred in more gentle formulations
Commonly used in:
Mild shampoos and body washes
Baby care products
Sensitive skin formulations
“Sulfate-free” labeled products
Higher-end personal care items
Suitable for leave-on products due to its milder nature
Often used in combination with other mild surfactants
Sodium Lauryl Sulfate (SLS):
Used in a wide range of products due to its strong cleansing power
Commonly found in:
Mass-market shampoos and body washes
Toothpaste
Dish soaps and laundry detergents
Industrial cleaners
Car wash products
Generally not used in leave-on products due to potential irritation
Often combined with other surfactants to balance performance and mildness
Factors influencing usage:
Product type: Gentle products favor olefin sulfonate, while strong cleansing products prefer SLS.
Target audience: Products for sensitive skin or babies are more likely to use olefin sulfonate.
Marketing claims: “Sulfate-free” or “gentle” products typically use olefin sulfonate instead of SLS.
Regulatory requirements: Some regions have restrictions on SLS usage in certain product types.
Formulation needs: SLS’s strong foaming properties make it preferred in products where foam is desired.
Trends in usage:
Increasing shift towards milder surfactants like olefin sulfonate in personal care products.
Growing consumer awareness has led to more “SLS-free” products in the market.
Some brands are reformulating traditional products to replace SLS with alternatives.
Formulation considerations:
Olefin sulfonate may require different thickening systems compared to SLS.
The choice between these surfactants can affect the need for additional conditioning agents in the formulation.
pH adjustments may be necessary depending on which surfactant is used.
It’s important to note that while these surfactants have their typical usage patterns, formulators often make decisions based on a combination of factors including performance requirements, cost constraints, and market positioning of the final product.
Welcome to our website! As a professional AOS (alpha olefin sulfonate) &SLS supplier, we are committed to providing customers with high-quality products and excellent services. Whether you are looking for AOS for detergents, personal care products, industrial cleaning agents, or other applications, we can meet your needs. We have rich industry experience and expertise to provide you with customized solutions. Please feel free to contact us, we look forward to working with you to jointly promote the success and development of your business.